DETERMINATION OF CRITICAL MICELLER CONCENTRATION OF SODIUM LARYL SULPHATE
AIM: To determine the critical micelle concentration of given surfactant (sodium lauryl sulphate) using a stalagmometer (surface tension method).
APPARATUS CHEMICALS: Stalagmometer, sodium lauryl sulphate (SLS). Given Mol. Wt. of SLS = 288.
PROCEDURE:
1. Fix a clean and dry stalagmometer in a vertical position. The Y tube fitted at the upper end should also be held in a fixed position.
2. Make two ink marks. A is the upper most on the upper stem and B is the lowermost on the lower stem to count the number of drops between these two marks. This fixes volume "V" of the liquid.
3. Suck the liquid above the mark A and see that the clips C and the screw type pinchcock "P" are tight enough not to allow the liquid to flow down.
4. Adjust the flow of liquid with P to give about 35-40 drops per mins. The flow should be such that the liquid drop is allowed to grow to its full size before it gets detached.
5. Determine the number of divisions (N) on the stem that correspond to 1 drop of the liquid.
6. Determine the number of drops of the liquid between the marks A and B by applying the necessary correction.
7. Determine the number of drops n for different concentrations of SLS as shown in table. Take 3 readings for each liquid.
OBSERVATIONS:
R.T. = …… °C
Sw = Surface tension of water = 72 dynes/cm
d = Density of solution = Density of water, since the solutions are very dilute = 1 gm/cc.
C = Conc. of SLS in mg/100 ml solution in D.W.
nw = Number of drops for water
n = Number of drops for any other liquid
dn = Difference in the number of drops of two consecutive solutions
dc = Difference in the concentration of two consecutive solutions.
GRAPH: Plot the graph of S.T. vs Conc. Draw the two "extreme" tangents to the curve obtained. The point of intersection of the two tangents gives the critical micelle concentration (CMC). Most of the time it is difficult to draw the tangents and hence the first derivative plot dn/dc vs conc. may be used to determine the CMC
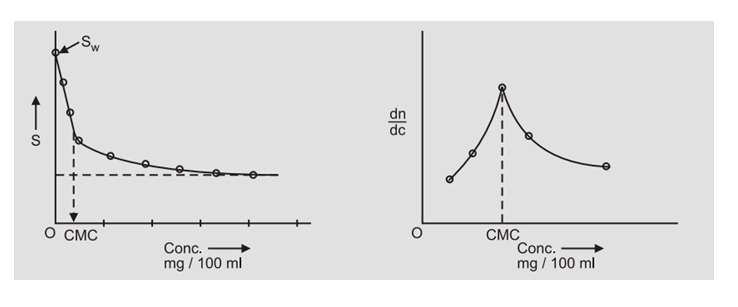
RESULT: The critical micelle concentration of sodium Lauryl sulphate is = ……… mg/100 ml